Background:
The median age at diagnosis for systemic AL amyloidosis is reported to be 64 years. A small number of patients are diagnosed at a much younger age, i.e., before 40 years. The characteristics and prognosis of these patients are not well known, and perhaps they require unique considerations due to the earlier onset of disease. A closer understanding of this patient population could help guide management approach and improve long term outcomes.
Aim:
In this study, we aimed to describe the presenting clinical features, hematologic profiles and outcomes of young patients with AL amyloidosis.
Methods:
Patients with systemic AL amyloidosis and age ≤ 40 years at diagnosis who presented to the Boston University Amyloidosis Center between 2000 and 2023 were identified from our prospectively maintained database of consented patients (ClinicalTrials.gov Identifier: NCT00898235). All patients had clinicopathological evidence of systemic AL amyloidosis, with positive Congo red staining of a biopsy specimen and evidence of a plasma cell dyscrasia. We collected clinical data both from the database and medical records of these patients. Overall survival (OS) rates were estimated using the Kaplan-Meier method, and hazard ratios (HR) computed from multivariable Cox proportional hazard models for OS.
Results:
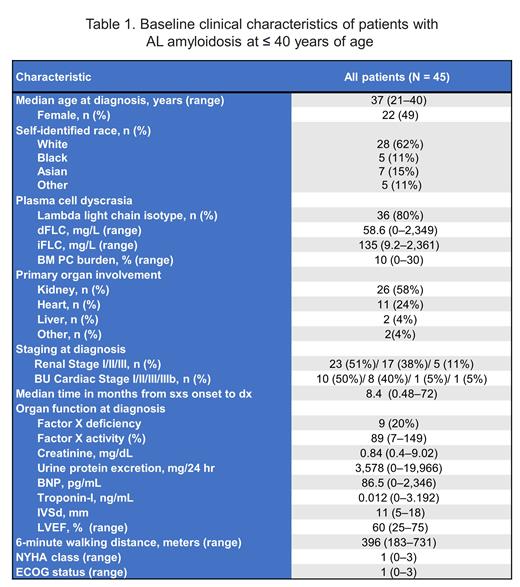
Of 1,722 patients with AL amyloidosis evaluated during the study period, we identified 45 (2.6%) patients who were diagnosed at ≤ 40 years of age. The median age was 37 years (range 21-40) and only 4 patients were ≤ 30 years of age. Presenting characteristics are shown in Table 1. Notably, this cohort had a median B-type natriuretic peptide of 87 pg/mL (range 0-2,346), troponin-I of 0.012 ng/mL (range 0-3.192), interventricular septum of 11 mm (range 5-18), and 6-minute walking distance (6MWD) of 396 meters (range 183-731). The median time from symptom onset to diagnosis was 8 months (range 1-72). The majority (n = 32, 71%) were treated with high-dose melphalan and stem cell transplantation (HDM/SCT) as frontline therapy. Meanwhile, 11 patients received non-SCT treatments, including melphalan/dexamethasone (7%), daratumumab-CyBorD (4%), CyBorD (2%), bortezomib/dexamethasone (2%), and dexamethasone alone (2%). Of the evaluable patients (n = 31), 39% achieved hematologic complete response to any regimen at 6-12 months following treatment.
With a medianfollow-up of surviving patients of 9.0 years, the median OS was 16.7 years (95% CI 11.6-16.9). Of the cohort, a total of 20 patients (44%) died. Patients treated with HDM/SCT had median OS of 16.9 years; meanwhile, those treated with non-SCT therapies had a median OS of 1.3 years. On multivariate analysis, time from symptom onset to diagnosis of >6 months (HR 2.21, p = 0.21) and 6MWD < 400 meters (HR 1.37, p = 0.66) were independently associated with increased risk of mortality.
Conclusions:
Young patients with AL amyloidosis who are diagnosed before 40 years of age have a prolonged survival if treated with intensive treatment with HDM/SCT if eligible. Longer time to diagnosis from symptom onset and 6-minute walk distance are important prognostic factors in this patient population.
Disclosures
Sanchorawala:Janssen, Alexion, Prothena, Celgene, Takeda, Abbvie, Regeneron, Pfizer, AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal